Biogeochemical models predict that ocean warming is weakening the vertical transport of nutrients to the upper ocean, with severe implications for marine productivity. However, nutrient concentrations across the ocean surface often fall below detection limits, making it difficult to observe long-term changes.
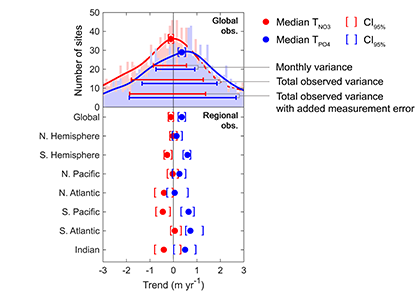
In a recent study in PNAS, we analyzed over 30,000 nitrate and phosphate depth profiles observed between 1972 and 2022 to quantify nutricline depths, where nutrient concentrations are reliably detected. These depths accurately represent nutrient supplies in a global model, allowing us to assess long-term trends. Over the past five decades, upper ocean phosphate has mostly declined worldwide, while nitrate has remained mostly stable. Model simulations support that this difference is likely due to nitrogen fixation replenishing upper ocean nitrate, whereas phosphate has no equivalent biological source.
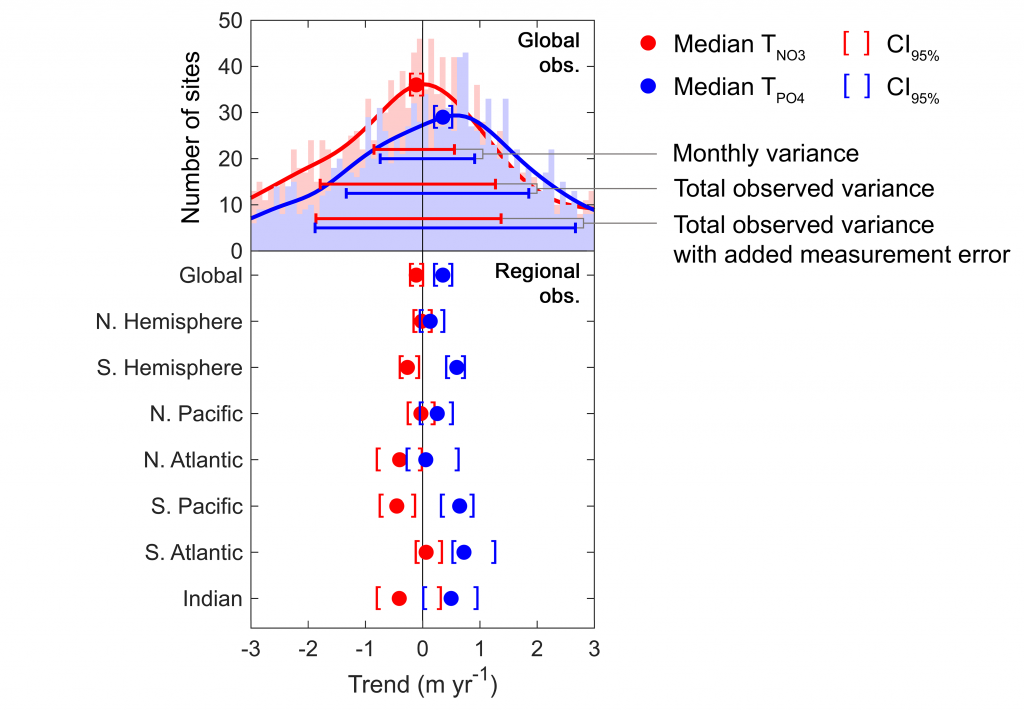
Figure caption: Five decades of global and regional nutricline depth data reveal declining phosphate-to-nitrate trends. Nutricline depths were defined based on threshold concentrations of 3 μmol kg−1 nitrate (TNO3) and 3/16 μmol kg−1 phosphate (TPO4). Site-specific trends were quantified for each unique pair of geographic coordinates where sufficient data was available (TNO3, n = 1,859 sites; TPO4, n = 1,641 sites). Shown are 95% confidence intervals (CI95%) calculated for each median trend by generating 10,000 bootstrap samples. The curves over the histograms depict the kernel densities. The sets of error bars from top to bottom are the interquartile ranges of TNO3 and TPO4 from a monthly climatology, the total observations, and the total observations with added measurement error.
These findings suggest that the ocean is becoming more limited in phosphorus. This decline could make phytoplankton less nutritious for marine animals. Fish larvae growth rates correlate with phosphorus availability in the ecosystem, so intensifying phosphorus limitation may greatly impact fisheries worldwide.
Authors
Skylar Gerace (University of California, Irvine)
Jun Yu (University of California, Irvine)
Keith Moore (University of California, Irvine)
Adam Martiny (University of California, Irvine)
@UCI_OCEANS