GEOTRACES and particles in the ocean
GEOTRACES is an international program to study the global marine biogeochemical cycles of trace elements and their isotopes (TEIs). The program’s guiding mission is to “identify processes and quantify fluxes that control the distributions of key TEIs in the ocean” (1).
Particles represent a key parameter for the GEOTRACES program because of their role as sources, sinks, and in the internal cycling of so many TEIs (1, 2). Particles in the ocean fall into two classes: 1. Those that have sources external to the system such as lithogenic material carried by atmospheric transport, river, or lateral transport from continental margin sediments; and 2. those that are produced internally in the system, primarily by biological production, but also by authigenic mineral precipitation (2).
External particle sources such as mineral dust deposition and sediment resuspension act as sources of dissolved TEIs when they partially dissolve in seawater. Conversely, dissolved TEIs are removed by active biological uptake or passive adsorption onto particles surfaces, followed by particle removal by aggregation and sinking. Indeed, the biological and abiotic interactions of dissolved TEIs with particles determine the residence time of a dissolved TEI.
In most open ocean basins away from ocean floor boundaries, external sources of particles are dwarfed by the much greater biological production and destruction of particles. Particle cycling in most open ocean basins is thus dominated by the biological pump, the processes by which suspended particles are produced by photosynthesis in the euphotic zone at the surface, and are then abiotically or biologically aggregated into larger particles that can sink into the abyss (3).
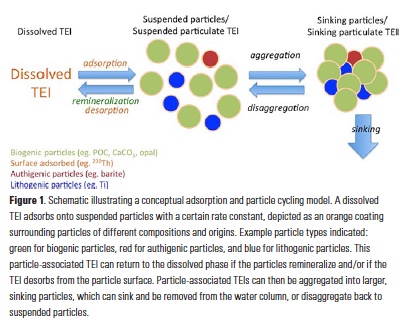
As particulate organic carbon (POC) cycles through processes such as aggregation, disaggregation, remineralization, and sinking (collectively referred to here as particle dynamics), other particle phases are swept along for the ride, including other major components such as biologically precipitated minerals (especially CaCO3 and opal), as well as lithogenic and authigenic particles, and scavenged TEIs adsorbed to the surfaces of other particles (Fig. 1).
In this article, I will briefly review the role of particle composition on the scavenging of TEIs.
Scavenging: A two-step removal process
Most adsorption of TEIs likely occurs onto small, suspended particles, which are usually more abundant, have more available surface area, and have a longer residence time in the water column than large, sinking particles. For TEIs to be removed from the water column, the suspended particles must then be aggregated into larger, sinking particles. There are thus two distinct steps for the removal of a dissolved TEI by scavenging: 1) adsorption onto suspended particle surfaces, followed by 2) removal via the aggregation of suspended particles onto larger particles that sink out of the water column. Fig. 1 shows a very simple schematic illustrating these basic processes. The adsorption step is governed by the affinity of a TEI for a particular particle surface, and the removal step is governed by the particle dynamics that package suspended particles into large, sinking aggregates, and are the focus of studies of the biological pump. The removal of TEIs by scavenging thus intimately links one of OCB’s scientific goals, the understanding of the biological carbon pump, to GEOTRACES’s mission to identify processes and quantify fluxes that control the distributions of key TEIs in the ocean.
Particle concentration and composition: horizontal and vertical variations
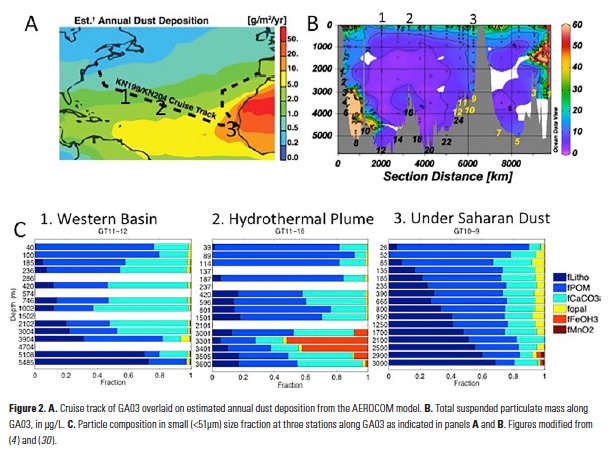
Particles collected in the ocean are a heterogeneous mixture of biogenic, lithogenic, and authigenic (precipitated in-situ) components. The relative proportions of these different components vary geographically and with depth. Fig. 2 shows the distribution of total particle concentration from GA03, the U.S. GEOTRACES North Atlantic Zonal Transect cruise in 2010/2011, as well as the changing composition of small (<51 mm) particles at three stations along the transect (4). Particle concentrations are highest at the surface and at the margins, where biological production is highest. It is clear that particulate organic matter (POM) dominates particle composition in the upper 100 m, making up more than 70% of the suspended particle mass at all three stations. The balance in the upper 100 m is mostly made of other biogenic components such as CaCO3 and opal, with a small contribution from lithogenic particles directly under the Saharan dust plume. At all stations, the inorganic components (everything except for POM) become relatively more important with depth as POM is remineralized. In the eastern half of the basin, lithogenic particles make up the largest fraction of particle mass, accounting for >50% of particle mass below 1500 m. In the western half of the basin, further from the Saharan dust source, lithogenic particles are not as important, and CaCO3 makes up the largest fraction (~50%) of particle mass between 500 – 3000 m. A special case is found in a station over the Mid-Atlantic Ridge, where iron oxyhydroxides from the hydrothermal plume make up ~50% of the particle mass. Iron and manganese oxyhydroxides are rarely dominant components of particle mass, except in special situations such as hydrothermal plumes, but may exert a particularly large influence on TEI adsorption (5, 6 ).
Studies suggest that particle composition may affect both the affinity of dissolved TEIs for adsorbing onto particle surfaces (2), and the vertical flux of particles from the water column (7-9). Horizontal and vertical changes in particle composition thus allow us to test hypotheses of the importance of particle composition on both steps in the scavenging of TEIs.
Effect of particle composition on adsorption of TEIs
The affinity of TEIs to particles has typically been characterized by a partition coefficient, Kd, which is calculated empirically as:
![]()
Prior to the GEOTRACES program, the effect of particle composition on TEI adsorption affinity had been studied in the field using sediments and sinking particles collected in sediment traps. The affinity of trace metals to marine sediments of different compositions varied: Some trace metals (Cs, Be, Sn, and Fe) had a higher affinity to sediments dominated by aluminosilicate clay minerals, and others (Ba, Cd, Zn, Mn, and Co) had a higher affinity to sediments enriched in Mn oxyhydroxides (10). In the water column, correlations between the partition coefficient of 230Th and particle composition in sediment trap particles from around the world have variously implied that the scavenging efficiency of 230Th is controlled by CaCO3 (11, 12), lithogenic material (13, 14), and/or Mn oxyhydroxides (15). Studies that span strong opal gradients across the Polar Front in the Southern Ocean show higher partition coefficients for 231Pa scavenging in areas of high opal content (11, 16). 231Pa is generally not as particle-reactive as 230Th in the open ocean, but is often removed with equal efficiency as 230Th in near-margin areas (e.g., 17), presumably because opal is more important in margin settings. The Arctic, on the other hand, displays the opposite 230Th/231Pa removal signal, with 231Pa removal less efficient relative to 230Th at the margins compared to the open ocean (18).
Since TEIs adsorb primarily onto suspended particles rather than sinking particles, studying the correlations between partition coefficients and suspended particles may resolve some of the discrepancies observed in the sediment trap studies (c.f., 2).
The GEOTRACES GA03 North Atlantic Zonal Transect has provided the first opportunity to investigate the correlation between partition coefficients of various TEIs and the particle composition of suspended particles in the ocean. Thus far, this has been done for 230Th and 231Pa partition coefficients, with Mn and Fe oxyhydroxides emerging as key controlling phases and opal having no controlling effect (5). The North Atlantic is very opal-poor (Fig. 2), so particles collected from more diatom-rich regions are needed to examine the potential of opal as a controlling phase. Other studies are underway to study the particle affinities of Hg (19), Po (20), and Pb (6) on this same North Atlantic transect. Subsequent U.S. GEOTRACES sections (GP16—Eastern Tropical South Pacific Zonal Transect and GN01—Western Arctic) will also have full ocean depth size-fractionated particle concentration and composition, allowing us to examine samples from different biogeochemical provinces, and hopefully expanding the range of particle compositions.
TEIs as tracers of scavenging rates and particle dynamics
The unprecedented data sets from GEOTRACES are also allowing us to estimate adsorption and desorption rate constants (Fig. 1) from inverse modeling of the observations of dissolved and particulate TEIs and particle concentrations (19, 21, 22). This gives us a kinetic view of the scavenging process to complement the empirically-derived partition coefficients, which are often viewed as representing equilibrium constants. Applying inverse modeling approaches to observations of the distributions of size-fractionated particles and particulate TEIs can also allow us to estimate rates of particle remineralization, aggregation, disaggregation, and sinking (21, 23). This approach requires only that a conceptual model relating the suspended and sinking particle size fractions be applied to observations of particle mass and particulate TEIs, and does not require knowledge about which specific physical or biological processes are responsible for particle transformations. For example, Fig. 1 illustrates a simple conceptual model in which a pool of suspended particles can be lost to the dissolved phase through remineralization, or by aggregation into sinking particles; conversely, sinking particles can sink, or can be disaggregated back into suspended particles. By assuming that particulate TEIs are simply part of the overall particle pool (e.g., a coating on organic particles in the case of radiogenic TEIs such as 230Th or as part of a lithogenic particle in the case of a TEI such as Ti) and thus are subject to the same rates of particle transformations as the major phases such as POC, we can apply the same conceptual model to observations of particle mass and to observations of particulate TEI to better constrain the rates of these transformations (23). As some of these rates such as aggregation and disaggregation are notoriously difficult to measure directly, these inverse approaches offer a way forward to quantify these important processes.
Particle composition and the biological pump
In addition to its effect on scavenging efficiency, particle composition has also been implicated as an important factor in the strength and efficiency of the biological pump. Several meta-analyses of global deep (>1000 m) sediment trap data showed strong correlations between POC flux and mineral flux (7-9), leading to the development of the “ballast hypothesis.” The mechanisms to explain the correlations, which are still being debated (24, 25), range from mineral protection of POC (8), mineral contribution to particle excess density (7), scavenging of mineral particles by POC (26), and minerals as proxies for particle packaging, POC lability, and ecosystem structure (9, 27-29).
Although the GA03 dataset is based on size-fractionated particle samples collected by in-situ filtration rather than sinking particles collected by sediment traps, we can nonetheless examine whether there is a correlation between POC and ballast minerals in small or large particle size fractions. We found that POC concentration in large (>51 mm) particles was not consistently correlated with any of the potential ballast minerals CaCO3, opal, and lithogenic particles (4).The lack of strong correlations within this regional dataset is consistent with the idea that ballast mineral correlations with POC may only emerge in global datasets that combine different biogeochemical provinces (25).
Outlook
The GEOTRACES program is not only rapidly expanding global observations of dissolved TEIs, but it is also the latest major program to systematically sample particle distributions since JGOFS and GEOSECS (2). These particle measurements are not only helping us understand the processes controlling TEI distributions, but the TEI measurements can also be used as tracers for quantifying key processes of particle cycling. Both GEOTRACES and OCB can benefit from the insights gained in each program.
Author
Phoebe J. Lam (Department of Ocean Sciences, University of California, Santa Cruz)
References
1. GEOTRACES, Scientific Committee on Oceanic Research, Ed. (Baltimore, Maryland, 2006).
2. C. Jeandel et al., Progress in Oceanography 133, 6 (4//, 2015).
3. C. L. De La Rocha, in Treatise on Geochemistry, H. Elderfield, K. K. Turekian, Eds. (Elsevier, 2003), vol. 6: The Oceans and Marine Geochemistry, pp. 83-111.
4. P. J. Lam et al., Deep Sea Research Part II: Topical Studies in Oceanography 116, 303 (6//, 2015).
5. C. T. Hayes et al., Marine Chemistry 170, 49 (3/20/, 2015).
6. E. A. Boyle et al., paper presented at the 2016 Ocean Sciences Meeting, New Orleans, LA, USA, 2016.
7. C. Klaas, D. E. Archer, Global Biogeochemical Cycles 16, 1116 (Dec 5, 2002).
8. R. A. Armstrong et al., Deep-Sea Research Part II-Topical Studies in Oceanography 49, 219 (2002).
9. R. François et al., Global Biogeochemical Cycles 16, (Oct-Nov, 2002).
10 . L. S. Balistrieri, J. W. Murray, Geochimica Et Cosmochimica Acta 48, 921 (1984).
11. Z. Chase et al., Earth and Planetary Science Letters 204, 215 (Nov 30, 2002).
12. Z. Chase et al., Deep-Sea Research Part II-Topical Studies in Oceanography 50, 739 (2003).
13. S. D. Luo, T. L. Ku, Earth and Planetary Science Letters 220, 201 (Mar, 2004).
14. M. Roy-Barman et al., Earth and Planetary Science Letters 286, 526 (2009).
15. M. Roy-Barman et al., Earth and Planetary Science Letters 240, 681 (2005).
16. H. J. Walter et al., Earth and Planetary Science Letters 149, 85 (1997).
17. R. F. Anderson, M. P. Bacon, P. G. Brewer, Earth and Planetary Science Letters 66, 73 (1983).
18. H. N. Edmonds et al., Earth and Planetary Science Letters 227, 155 (Oct, 2004).
19. C. H. Lamborg et al., Philos T R Soc A, (accepted).
20. Y. Tang et al., paper presented at the 2016 Ocean Sciences Meeting, New Orleans, LA, USA, 2016.
21. O. Marchal, P. J. Lam, Geochimica Et Cosmochimica Acta 90, 126 (2012).
22. P. Lerner et al., Deep Sea Research Part I: Oceanographic Research Papers 113, 57 (7//, 2016).
23. P. J. Lam, O. Marchal, Annual Review of Marine Science 7, 159 (2015).
24. P. Boyd, T. Trull, Progress in Oceanography 72, 276 (2007).
25. J. D. Wilson, S. Barker et al., Global Biogeochemical Cycles 26, GB4011 (2012).
26. U. Passow, Geochemistry Geophysics Geosystems 5, (Apr 6, 2004).
27. P. J. Lam et al., Global Biogeochem. Cycles 25, GB3009 (2011).
28. S. A. Henson et al., Global Biogeochem. Cycles 26, GB1028 (2012).
29. S. Z. Rosengard et al., Biogeosciences 12, 3953 (2015).
30. D. C. Ohnemus, P. J. Lam, Deep Sea Research Part II: Topical Studies in Oceanography 116, 283 (6//, 2015).



